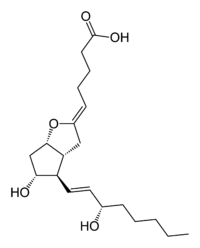
Latest news on Prostacyclin analogues
The era of therapy targeted specifically to the correction of mechanism aberrations underlying PAH began with FDA approval of epoprostenol sodium in 1995, and its commercial availability in 1996 for cure of WHO III or IV patients with PAH or IPAH related with sceroderma. The prime objective of treatment with epoprostenol or any of the prostacyclin analogues is to compensate for the deficiency of endogenous prostacyclin analogues is to compensate for the deficiency of endogenous prostacyclin in patients with PAH. In views of its short half life and not being absorbed through the gastrointestinal tract, epoprostenol requires continuous intravenous administration.
A randomized 12 week study of 81 IPAH patients with WHO functional class III and IV symptoms demonstrated improved six minute walk distance 113 m compared to untreated and pulmonary hemodynamics in treated patients and suggested a survival benefit.
Subsquent clinical trials confirmed prolonged survival with respect to historical controls in the NH registry using intravenous epoprostenol. Among patients with predominantly scleroderma associated PAH, intravenous epoprostenol significantly improved six minute walk distance and pulmonary hemodynamics during 12 weeks of therapy; though a survival effect in this short time frame was not demonstrated. Additional uncontrolled reports have indicated similarly salutary outcomes of intravenous epoprostenol in APAH related to connective tissue disease congenital heart disease, HIV infection and portopulmonary hypertension.
Administration of intravenous epoprostenol is complex requiring that patients take an active role in their own management, including aseptic management of their indwelling central venous catheter, daily sterile preparation of the drug, use of the ambulatory infusion pump, and awareness of trouble shooting techniques in the event of problems.
Most patients experience optimal benefit at a dose of 25-45 ng/kg/min after gradual incrementation of the dose over 6-12 months following initiation at 2-6ng/kg/min. Some patients require doses out side of this spectrum depending on level of symptomatic benefit or occurrence of adverse effects, such as flushing, rash, jaw pain, leg discomfort,loose stools or headache. Excessive dosing can produce fatigue and high output cardiac failure. Additional concerns include infection of the central venous catheter, sepsis or sudden cessation of the infusion causing rebound PH and possible death.